"If entropy increases over time," Zac interjected, "how come I don't melt or dissipate into a fine entropic mist? What's keeping my body together? My body is in a very low-probability state, right? Like, there are a very limited number of configurations my cells can be in to make up my particular functioning body. If the cells in my face were to move around too much, I wouldn't even look like Zac anymore, let alone be alive. My body is in such a particular state."
"That's a great question," I said. "And very relevant to solving this riddle. Let's imagine that I have a glass of water, and I drop some black ink into that water. Over time, the ink will disperse until it evenly mixes with the other water particles."
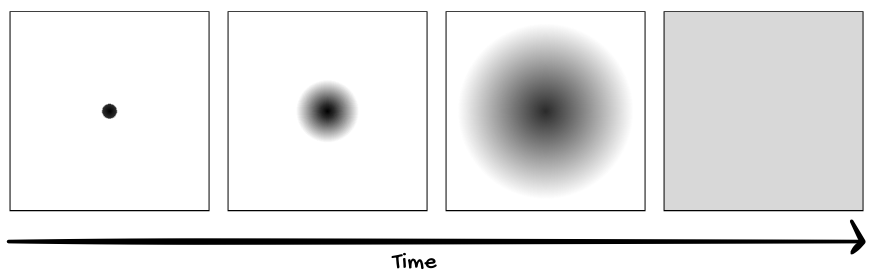
"Is that ink drop alive?" I asked.
"No," Zac replied.
"Why not?"
"Because it's not living and breathing and eating and all that."
"True. But that's not why," I said. "If you were to watch the ink spread out into the water and then gather itself back up into a ball, or a square, or a star shape, would you say the ink is conscious?"
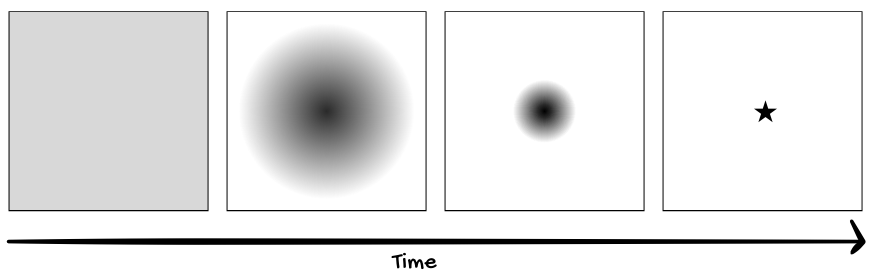
"Yeah, I guess. It's like David Bohm's electrons, right? The electrons collectively organized themselves into specific states. That's why Bohm thought the electrons seemed alive."
"Yep, that's exactly it," I said. "Here's what separates a conscious system from an unconscious system: a conscious system resists entropy. From a mathematical point of view, a conscious system moves from states of high probability to low probability.
Think of all the potential places we could find an ink molecule in that glass of water. The probability of finding the ink molecules arranged in a perfect star is very low. The probability of finding the ink molecules arranged in any number of evenly-distributed states is much higher."
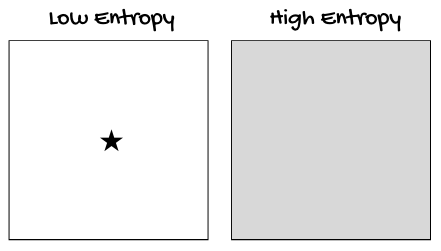
"So," I continued, "let's recap before moving on to the next point. All systems will naturally move from low probability (low entropy) states to high probability (high entropy) states. In other words, things move from being something 'in particular' to being evenly distributed and spread out in the system — like a drop of ink falling into a glass of water and dispersing itself evenly throughout the system.
Conscious systems do the opposite. They resist entropy. They adapt to their environment and move from high probability to low probability states. If you overheat, your body will cool you down. If you get too cold, your body will heat you up. Your body needs to exist at a specific temperature in order to keep itself in a particular state. This process is like pointing a cooling device on an ice cube, so it remains in a particular state and resists the natural inclination to melt. Like the ice cube, if your body didn't resist entropy, you would 'melt' — or decompose — and die. Make sense?"
Zac nodded.

